«Enteronormin» Registration Certificate
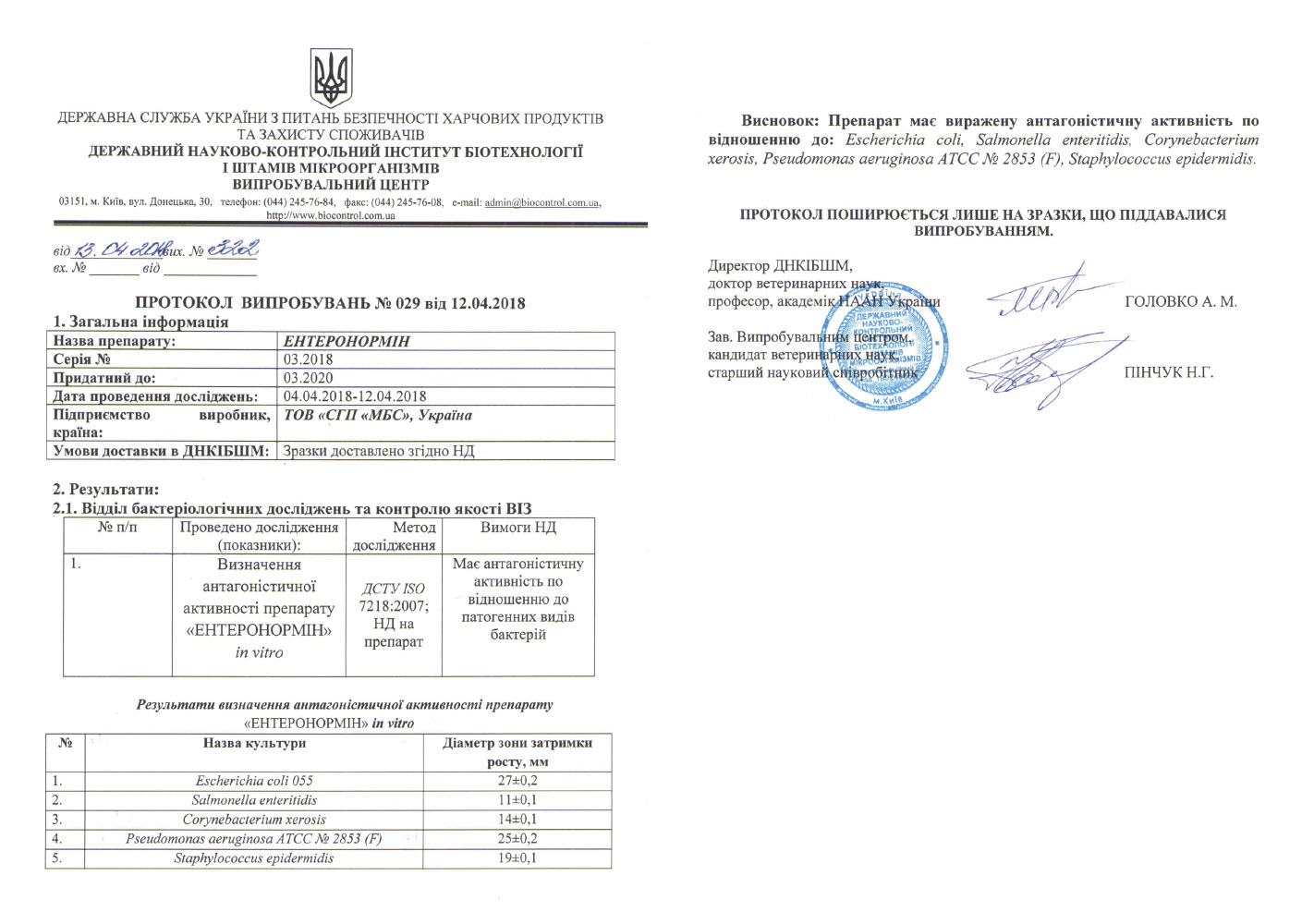
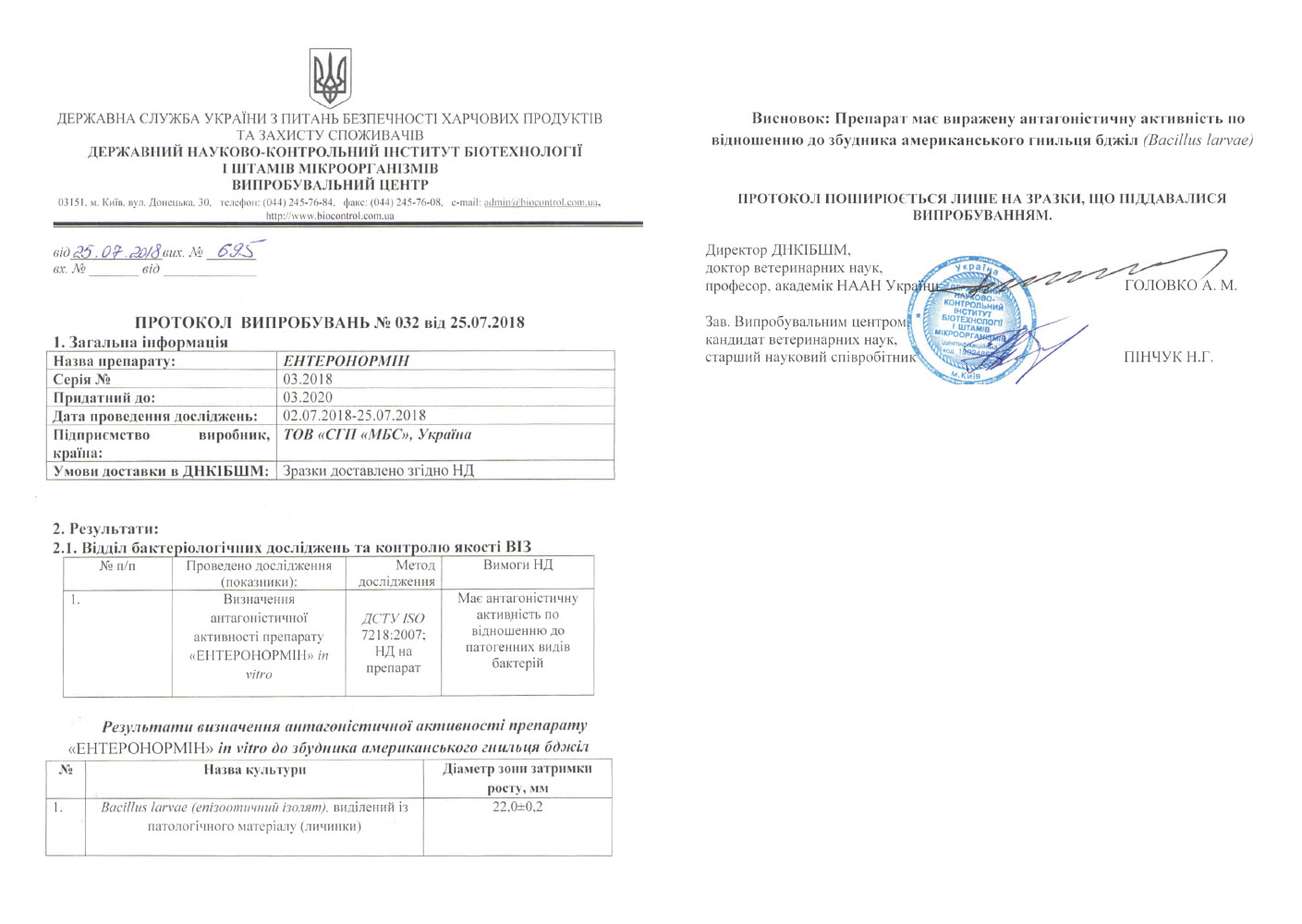
According to the laws of Ukraine «On Veterinary Medicine», a veterinary drug is allowed to be used on the territory of Ukraine following passing state registration. On the basis of an expert opinion, the veterinary drug, which received a positive decision, is entered into the register and receives a registration certificate.
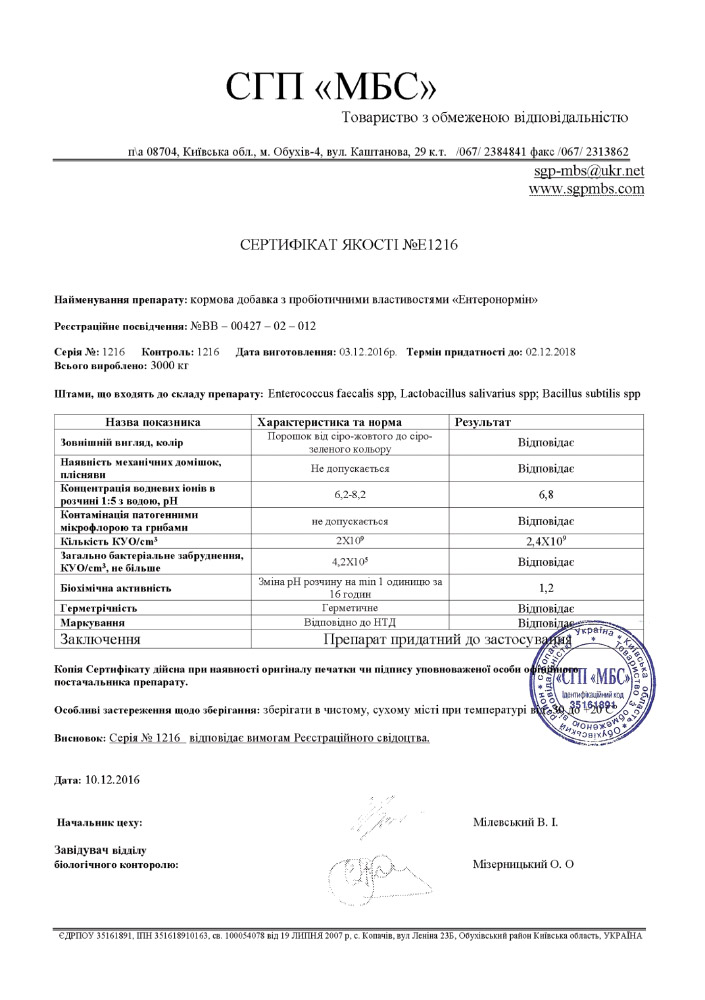
Product Quality Certificate
Each batch of manufactured products is checked for compliance with the requirements of the registration certificate. During check special attention ai paid to the chemical, biological and physical indicators of the drug. Only after a positive result of compliance, products are allowed for sale.
ISO 9001:2008 Quality Certificate
The international certification standard of compliance with ISO 9001 is one of the main indicators which consumers pay attention to when choosing a products manufacturer. The certificate confirms the high degree of organization of the system of internal business processes that monitor product quality.
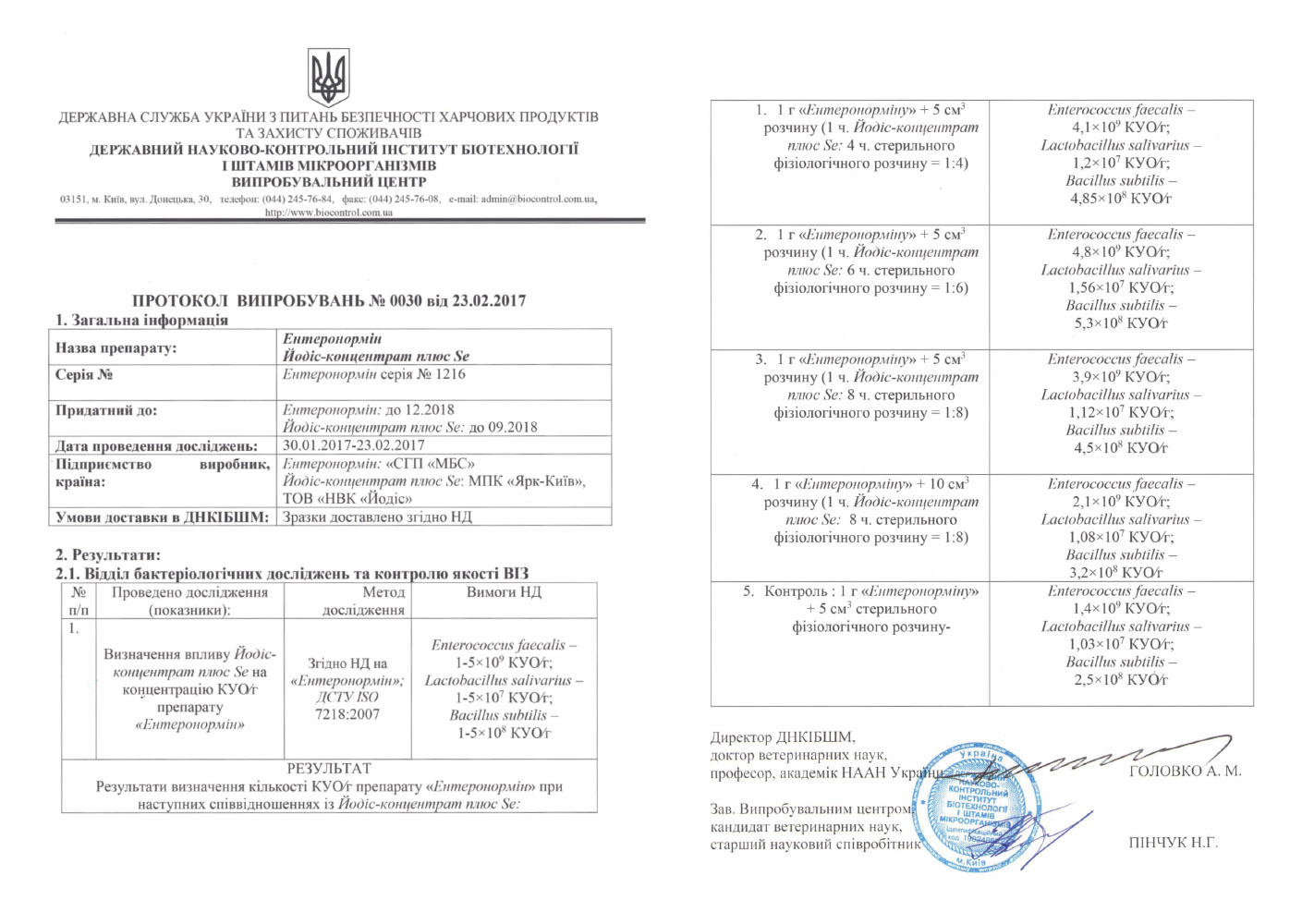
The effect of «Jodis+Se» on CFU/g concentration of the drug «Enteronormin»
Complex drug «Enteronormin» with an aqueous solution of «Jodis+Se» effectively uses the components ratio. The best result was achieved under conditions of 1 g of «Enteronormin» + 5 cm³ of solution (1 h. Jodis plus Se : 6 h. Sterile saline = 1:6).